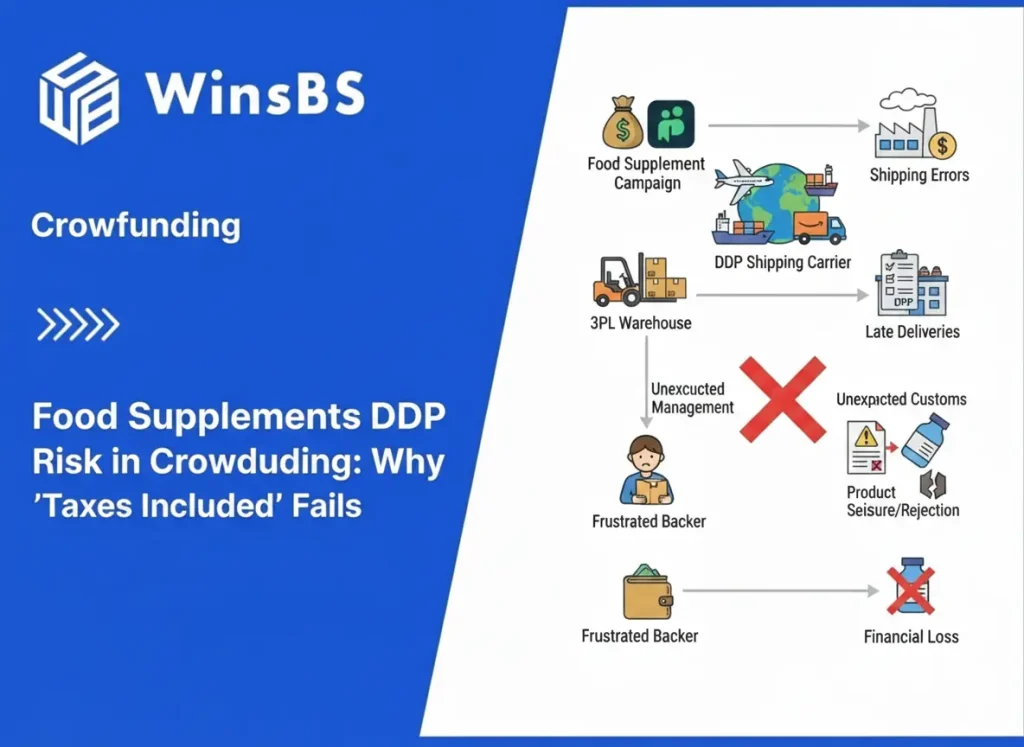
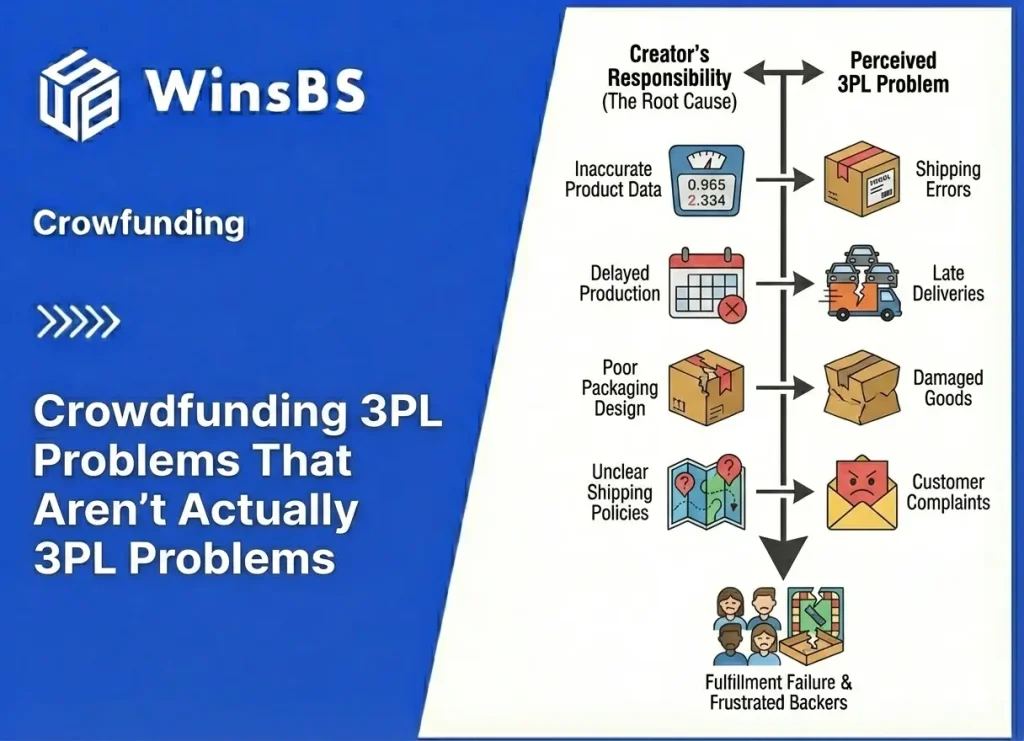
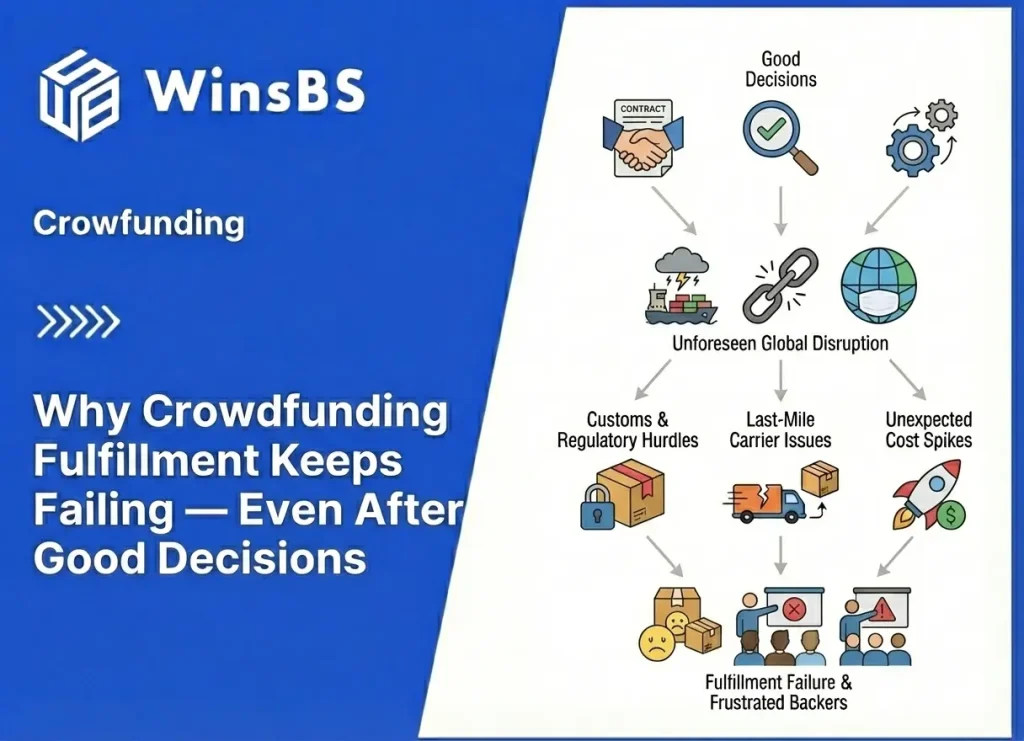
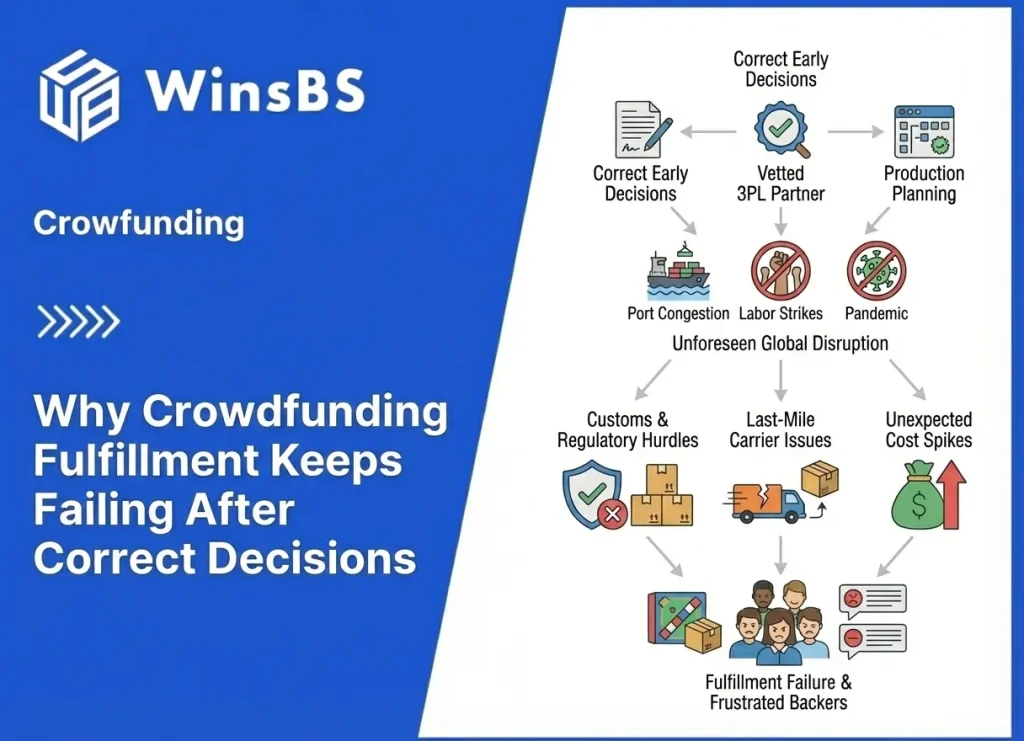
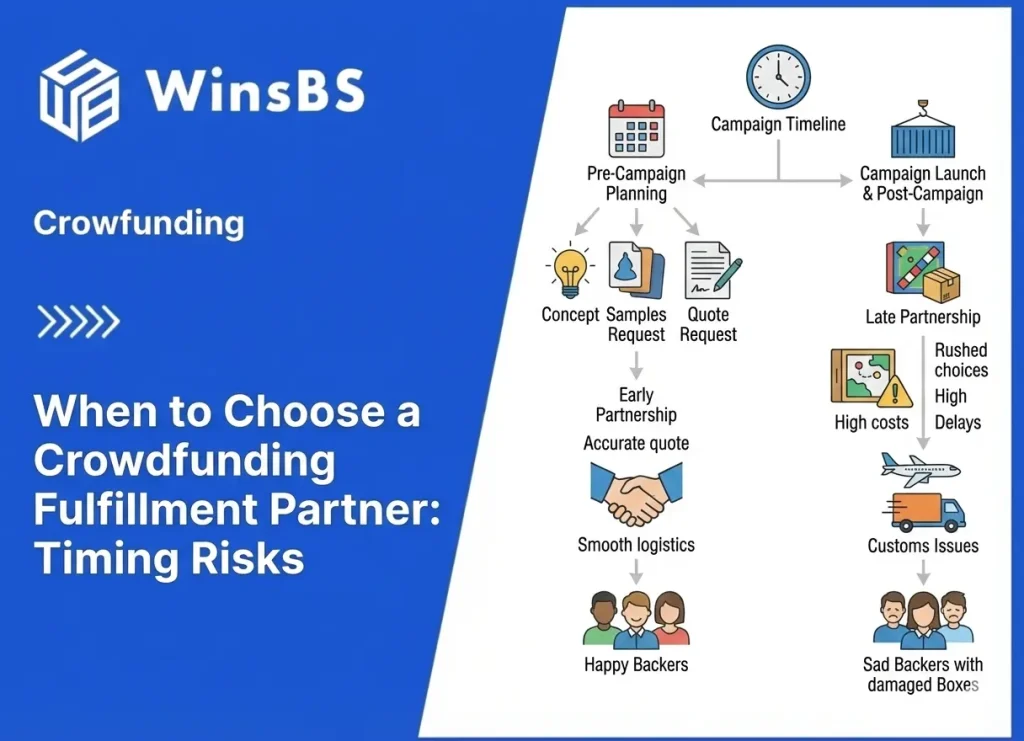
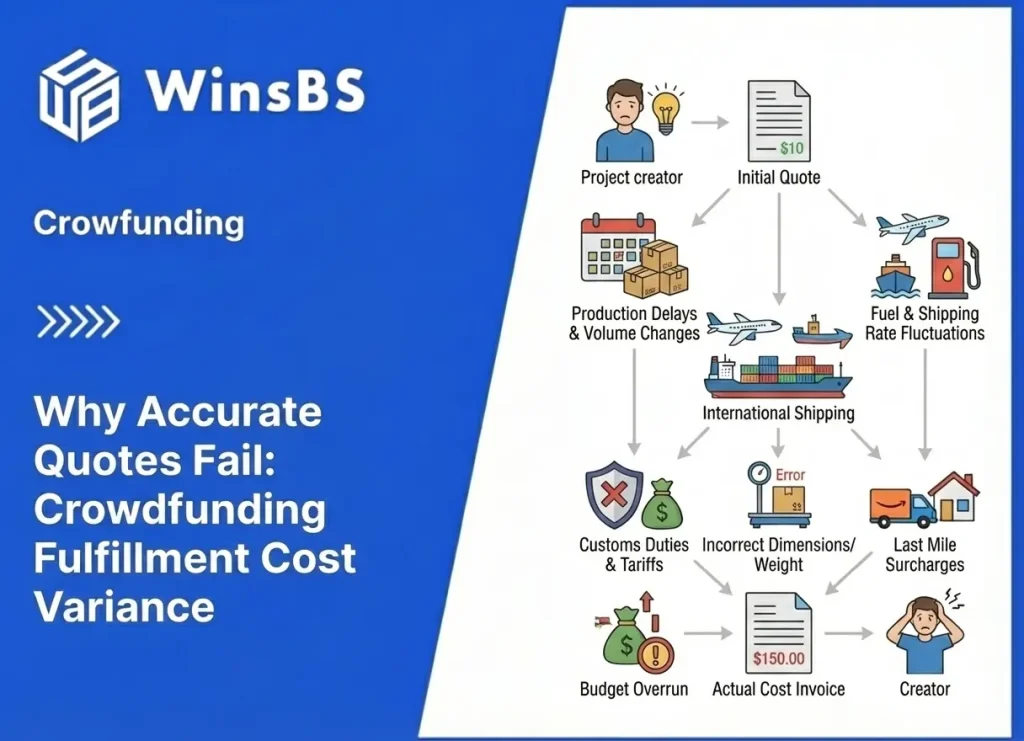
Food Supplements DDP Risk in Crowdfunding: Why “Taxes Included” Fails
Food Supplements DDP Risk in Crowdfunding Fulfillment Why “Taxes Included” Fails When Ingredients and Claims Aren’t Fixed WinsBS Fulfillment – Maxwell Anderson Updated January 2026 Table of Contents Key judgment: why DDP fails before shipping 1. Why food supplements are most often misjudged in crowdfunding 2. The three intuitive assumptions that fail under regulatory review 3. How regulatory systems actually define food supplements 4. Why DDP becomes structurally unstable for supplements 5. Country differences are not “strictness,” but entry gates 6. Why responsibility becomes non-transferable once problems appear 7. When DDP can work — and why these conditions are rare 8. Why crowdfunding timelines conflict with regulatory timelines 9. What this means before you commit to DDP Key judgment Food supplements are repeatedly misjudged in crowdfunding because they feel like “normal consumer goods.” Regulatory systems do not treat ingestible products that way. Once ingestion and health-related claims are involved, the category shifts into a compliance-first domain where admissibility determines outcomes long before duty payment matters. DDP (“taxes included”) can prepay duties and taxes, but it cannot make an inadmissible product admissible. When a campaign commits to DDP while ingredients, dosage, or label claims are still moving, DDP becomes structurally unstable: it locks responsibility at the exact moment the product identity is least stable. The failure often appears later as a “shipping problem,” but it begins earlier as a product-definition problem. A small post-campaign change that looks minor to backers can materially change how a supplement is treated at the border or in-market. 1. Why Food Supplements Are Most Often Misjudged in Crowdfunding Food supplements feel familiar. Creators have used similar products, seen them sold on major marketplaces, and watched countless brands ship them cross-border. Compared with categories that obviously “look regulated,” supplements can feel routine. That familiarity creates confidence. The problem is not that creators ignore rules. The problem is that they use the wrong mental model. Retail normality is not regulatory normality. A product being common in commerce is not evidence that it is stable under regulatory definitions. In the United States, the “dietary supplement” category is clearly defined by statute, placing direct responsibility on companies to assess safety and finalize labeling before marketing — a core principle the FDA continues to highlight in its ongoing guidance. Explore the FDA’s current overview of dietary supplements and regulatory responsibilities for firms. In the EU, the framework splits responsibilities: one directive sets the basic rules for food supplements as a product category, while a separate regulation strictly governs nutrition and health claims. This division alone disrupts many crowdfunding assumptions, since category approval and allowable claims don’t always align. See Directive 2002/46/EC on food supplements and Regulation (EC) No 1924/2006 on nutrition and health claims. Crowdfunding amplifies this mismatch because it commits to delivery terms while the product is still evolving. In established trade, supplements that ship reliably do so under stable formulas, stable labels, and stable claims. In crowdfunding, those elements often remain negotiable after funding. 2. The Three Intuitive Assumptions That Fail Under Regulatory Review Most supplement crowdfunding failures begin with three intuitions that feel reasonable in consumer commerce but fail under regulatory review. The first intuition is: “It’s just vitamins, herbs, protein, or probiotics — it’s not dangerous.” Regulatory control is not limited to “dangerous goods.” Ingestible products are controlled because they can be misleading, adulterated, misbranded, or positioned as medicines through claims. The question is not whether the product feels safe. The question is whether its identity and labeling fall cleanly inside the lawful category you are using to ship it — precisely the framework explained in the FDA’s detailed questions and answers on dietary supplements under DSHEA. FDA Questions and Answers on Dietary Supplements (DSHEA framework). The second intuition is: “Similar products sell on Amazon, so ours will be fine.” Marketplace presence is not proof of regulatory stability. Many products exist in commerce under inconsistent labeling quality, inconsistent claim discipline, and inconsistent ingredient documentation. Crowdfunding turns “inconsistency” into “failure” because you commit to thousands of cross-border deliveries under one promise. The third intuition is: “DDP solves it because taxes are prepaid and someone else handles the import.” DDP can change who pays and who arranges clearance, but it does not change what the product is. If the product’s admissibility is questioned, payment does not resolve the hold. DDP is a payment structure, not an admissibility guarantee — as the U.S. Department of Commerce clearly outlines in its practical guide to Incoterms. U.S. Department of Commerce — Know Your Incoterms (DDP overview). These assumptions appear “true” in mature supply chains because mature brands typically freeze formula and claims before scaling cross-border delivery. Crowdfunding reverses that sequence. 3. How Regulatory Systems Actually Define Food Supplements Supplements are not evaluated as “a product type people buy.” They are evaluated as a defined ingestible item with a defined composition and a defined label-claim posture. In practice, systems define supplements through the interaction of ingredients, dosage, intended-use framing, and claims language. In the U.S., “dietary supplement” is defined in statute. That definition does not magically make every ingestible “a supplement.” It describes the category boundaries that your product must actually fit — laid out in the legal text at 21 U.S.C. § 321 — Definitions (including dietary supplement definition). Manufacturing and handling expectations for supplements also sit inside formal compliance frameworks, including current good manufacturing practice requirements specific to supplements. This matters operationally because once a campaign scales, any mismatch in traceability, lot identity, or label control becomes a systemic exposure rather than a one-off mistake. The FDA’s small-entity compliance guide walks through these requirements in clear, practical detail. 21 CFR Part 111 — cGMP for dietary supplements and FDA Small Entity Compliance Guide for 21 CFR Part 111. Note that even as recently as mid-2025, the FDA released new educational videos and fact sheets to help companies better navigate the New Dietary Ingredient (NDI) notification process, underscoring how pre-market safety evaluations remain a key focus for certain ingredients. FDA’s latest